La cohorte de patients, gérée par les différentes associations et traitée par Bleu de méthylène semble indemne de syndromes grippaux et de Covid 19. Ceci peut être le simple fait du hasard ou plus probablement lié au Bleu de méthylène.
Nous avons soumis l’article ci-dessous à la revue « Substantia » de l’université de Florence. Ce papier a été accepté le jour même. Nous avons aussi rédigé un protocole en français pour tester l’efficacité de cette démarche.
La Chloroquine a été synthétisée en 1934 à base de Bleu de Méthylène dont on reconnait clairement deux des trois cycles.
[sg_popup id= »11703″ event= »click »]Traduire en français[/sg_popup]
Marc Henry1*, Mireille Summa2, Louis Patrick3, Laurent Schwartz4
1 Université de Strasbourg, Chimie Moléculaire du Solide, Institut Le Bel, Strasbourg.
2 Ceremade, Université Paris Dauphine
3 Association Espoir Métabolique
4 Assistance Publique des Hôpitaux de Paris, Paris, France.
Abstract
We report the case of a cohort of 2500 French patients treated among others with methylene blue for cancer care. During the COVID-19 epidemics none of them developed influenza-like illness. Albeit this lack of infection might be by chance alone, it is possible that methylene blue might have a preventive effect for COVID-19 infection. This is in line with the antiviral activity of Chloroquine, a Methylene blue derivative.
Both Chloroquine and Methylene blue have strong antiviral and anti- inflammatory properties probably linked to the change in intracellular pH and redox state.
Keywords: COVID-19, cancer, methylene blue, metabolic treatment
Introduction
Europe has been recently been hit by an epidemic of COVID-19. We report a cohort of patients treated for cancer in France. This cohort is managed by an association (Espoir Metabolique) and is a cancer support group. There are 2500 patients all at high risk for sepsis because of concomitant chemotherapy. One of us has interviewed (by telephone and by e mail) these patients to register the cases of COVID-19. As of March 27th, 2020, there were no cases of registered COVID-19 or of flu–like syndroms. These patients were treated by a combination of standard therapy and α-lipoic acid (800 mg twice a day), hydroxycitrate (500 mg three times a day) and methylene blue (75 mg three times a day) as well as a low carb diet.
There were 52% women against 48% men. The most prevalent cancer type were breast cancer (40 %), lung (20%), prostate (10%), uterine (10%), colon (8%), liver (6%) miscellaneous (6%). Albeit this lack of influenza-like illness might be by chance alone, it is possible that one of these molecule might have prevented viral infection. Herein, we present a short scientific survey of what is currently known about the SARS-Cov-2 virus, hydroxychloroquine treatment as well as biochemical properties of methylene blue that was called once upon a time a “magic bullet” for healing a wide range of diseases.
Background
Coronaviruses (CoVs) are quite common viruses that are generally related in humans to the upper respiratory tract family of disorders. They may trigger asthma in children and adults and severe respiratory disease in the elderly. They could also be responsible of pneumonia end bronchiolitis infections in the infant and child population. The first human coronaviruses was discovered in 1965 and named B814.1 Shortly after this discovery, other coronaviruses were described that caused disease in multiple animal species, including, rats, mice, chickens, turkeys, calves, dogs, cats, rabbits and pigs.2 In the late 1960s, two major human strains were studied HCoV-OC43 (“OC” meaning that they could be grown in organ cultures such as mouse brain) and HCoV-229E (“E” meaning that such viruses were ether-sensitive suggesting that they required a lipid-containing coat for infectivity), a strain that could be grown in tissue culture directly from clinical samples. Epidemiologic studies found that coronaviruses were endemic in humans, being responsible for 5-10% of all upper and lower respiratory tract infections associated to a quite low pathogenicity. But the situation changed in 2002-2003 after the discovery in Southern Asia of a new respiratory illness, termed Severe Acute Respiratory Syndrome (SARS), which was able to spread quickly in 29 countries of America, Asia and Europe causing 774 fatalities and resulting in a mortality rate of 9%.3 This SARS-CoV was responsible for a loss of $40 billions in economic activity. Phylogenetic studies seemed suggesting a bat origin for this new SARS-CoV virus with an entry in the human population probably relayed by Himalayan palm civets. The strange thing was that 40% of wild animal traders and 20% of individuals, who slaughter animals were seropositive for SARS, without manifestation of any symptoms, meaning that the real causes of the disease remain still nowadays quite unclear. Infection control policies were able to halt the epidemics in 2004.
But the same year, a new strain named HCoV-NL63 was identified in the Netherlands from an infant with bronchiolitis, followed by a new strain HCoV-HKU1 in 2005 from a patient with pneumonia in Hong Kong. These new strains were however not able to trigger new epidemics in the human population. This was however not the case of the MERS-CoV (Middle East Respiratory Syndrome Coronavirus) isolated in 2012 from a patient with pneumonia in Saudi-Arabia that was able to spread in 21 countries with almost 600 related deaths and a mortality rate of ≈ 40%. Again, bats were suspected of being at the origin of the virus, but with a new intermediate that were dromedary camels as natural hosts. It is worth noticing that since 1965, HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1 and MERS-CoV are commonly circulating in the human population causing general respiratory illness and cold symptoms during winter and spring months in healthy individuals and more severe disorders in the immuno-compromised and the elderly. We are now facing a new terrible challenge, with the emergence in late November 2019 of a new strain names SARS-CoV-2 in Wuhan, Hubei province, China. Concerning the origin of the virus, genomic studies have shown that Malayan pangolins (Manis javanica) illegally imported into Guangdong province contain coronaviruses similar to SARS-CoV-2 with as usual bats serving a reservoir hosts.4 Another possibility could be natural selection in humans following zoonotic transfer. But, genetic data irrefutably show that SARS-CoV-2 is not derived from any previously known virus backbone, meaning that it is improbable that it emerged after laboratory manipulation of a related SARS-CoV-like coronavirus. Nowadays, CoVs belong to the Nidovirales order split into two subfamilies (Coronavirinae et Torovirinae) and further subdivided into four groups: α-CoVs (HCoV-229E and HCoV-NL63), β-CoVs (OC43, HKU1, SARS, MERS), γ-CoVs and δ-CoVs.
Genome and structure
SARS coronaviruses are medium-sized RNA viruses, holding inside an oily shell a 30 kilobases long single stranded RNA-genome, positive in sense, with a 5’ cap structure ending with a 3’ poly-adenylated tail. The virions appear to spherical in shape with a diameter of about 125 nm with several club-shape spike projections emanating from the oily surface and conferring to the virus its corona-like aspect (figure 1).
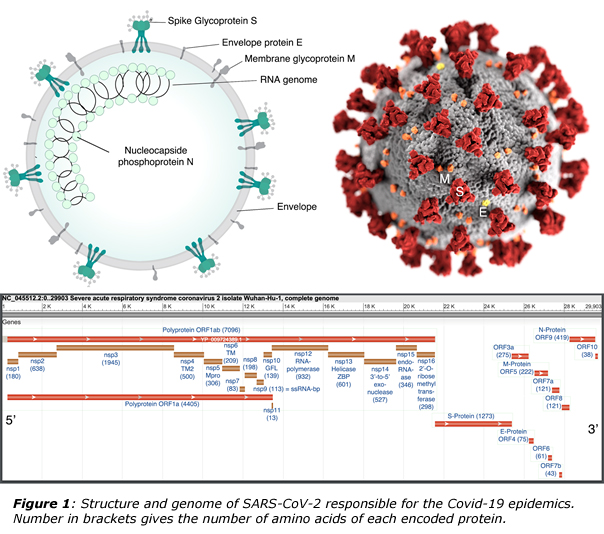
The overall packing of the coronavirus genome is 5’ – leader – UTR – Replicase (ORF1ab) – S (Spike) – E (Envelope) – M (Membrane) – N (Nucleocapsid) – 3’UTR – poly (A) tail with accessory genes interspersed within the structural genes at the 3’ end. UTR corresponds to untranslated regions. The replicase gene occupying two-thirds of the genome encodes for nonstructural proteins (Nsps) through two polyproteins (pps) 1a (coding for nsps1-11) and 1ab (coding for nsps1-16) with the following identified functions:4
– Gene nsp1 promotes cellular mRNA degradation and blocks host cell translation resulting in blockage of innate immune response.
– Gene nsp2 have currently no known function.
– Gene nsp3 encodes for a large multi-domain trans-membrane protein with ubiquitin-like and acidic domains that interacts with N-protein, ADP-ribose-1’’-phosphatase (ADRP) activity that promotes cytokine expression, papain-like protease (PLPro) with deubiquitinase domain is responsible for cleavage at nsp1/2, nsp2/3 and nsp3/4 boundaries and blocks host innate immune response.
– A potential trans-membrane scaffold protein playing an important role for proper structure of double-membrane vesicles (DMVs) in encoded in gene nsp4.
– A serine type main protease (Mpro) in nsps5 gene is responsible for all cleavage events not mediated by PLPro.
– Genes (nsp7, nsp8) encodes for two hexadecameric complexes that may act as processivity clamp for RNA polymerase.
– A RNA binding protein is encoded in gene nsp9.
– Gene nsp10 encodes for a cofactor that forms heterodimer with nsp16 and nsp14 for stimulating activity of the corresponding proteins.
– Gene nsp11 from pp1a extended into pp1b becomes nsp12 encoding the RNA-dependent RNA polymerase (RdRp) that duplicates viral RNA. The recombination ability of coronaviruses during viral evolution is tied to the switching ability of RdRp during replication.
– A RNA helicase with RNA 5’-triphosphatase activity insures unpackage of the viral genome (gene nsp13).
– Gene nsp14 encodes an Exoribonuclease (ExoN) that insures replication fidelity (proofreading of the viral genome) with N7-methyltransferase (N7-MTase) activity for adding 5’ cap to viral RNAs.
– A viral endoribonuclease (NendoU) with unclear function is encoded in gene nsp15 and is a genetic marker (together with nsp14-ExoN) for the order Nidovirales.
– Finally, gene nsp16 encodes for a 2’-O-methyltransferase (2’-O-MT) that shields viral RNA from MDA5 (melanoma differentiation associated protein 5) recognition.
Once cleaved, genes behave as mRNA for the ribosomal units of the infected cell for producing the set of Nsps that are able to self-assemble into a replicase-transcriptase complex (RTC) providing a suitable environment for producing both genomic and sub-genomics RNAs. The role of sub-genomic RNAs is to serve as mRNAs for the structural and accessory genes which resides downstream of the replicase polyproteins. Following replication and sub-genomic RNA synthesis, the viral structural proteins S, E and M are translated and inserted into the endoplasmic reticulum (ER) for further processing by the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). After encapsidation of the viral genome by the N-proteins in the ERGIC, budding with viral structural proteins (first with M and E, then with S later on) leads to mature virions. Following assembly, virions are transported to the cell surface in vesicles for further release by exocytosis.
If some S-proteins remain outside the virions, they may also transit towards the surface for mediating cell-cell fusion between infected cells and adjacent uninfected cells, leading to giant multinucleated cells, responsible for virus spreading without detection or neutralization by virus-specific antibodies. Accordingly, the primary determinant for infection of a cell by coronaviruses is the attachment of the virion through non-covalent interactions of protein-S with a suitable receptor. For human coronaviruses it is known that HCoV-OC43 binds to N-acetyl-9-O-acetylneuraminic acid, HCoV-HKU1 binds to O-acetylated sialic acids, while HCoV-NL63 and SARS-CoV bind to heparin sulfate proteoglycans.5 After binding to the cell, coronaviruses use a broad variety of fusion receptors: aminopeptidase N (APN) for HCoV-229E, human leucocyte antigen molecule (HLA class I) or sialic acids for HCoV-OC43, angiotensin-converting enzyme 2 (ACE2) for HCoV-NL63 and SARS-CoVs and dipeptyl-peptidase (DPP4) for MERS-CoV. The binding receptor of HCoV-HKU1 remains unknown. ACE2 receptors are expressed by epithelial cells of the lung, intestine, kidney and blood vessels, with a substantial upregulation in patients with type 1 or 2 diabetes or hypertension, who are treated by ACE inhibitors and angiotensin II type-I receptor blockers (ARBs).6 Increased expression of ACE2 is also observed by thiazolinidiones and ibuprofen. It has thus been inferred that people using ACE2-stimulating drugs may have a higher risk of developing severe and fatal COVID-19.
Chloroquine and hydroxychloroquine
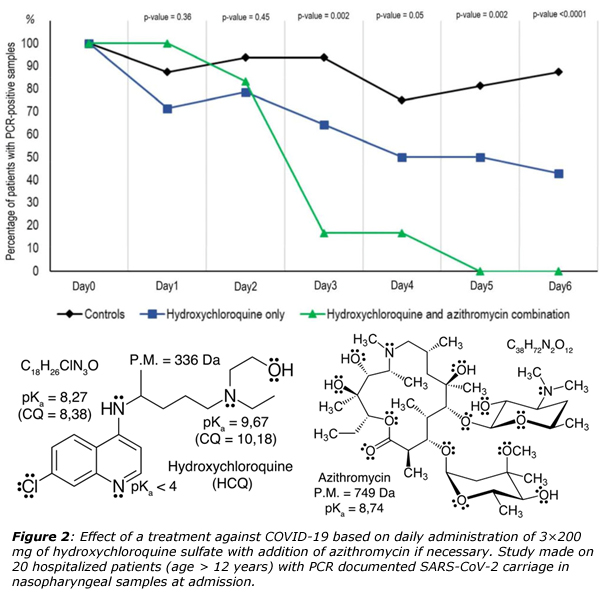
Quite recently, a French team led by Pr. Didier Raoult in Marseille, has reported that is was possible healing in less than a week COVID-19 patients after administration of an anti-malaria drug, hydroxychloroquine (HCQ) and an antibiotic, azithromycin (figure 2).7 It is worth noticing that antibiotics are generally of no use against viruses, but could be nevertheless useful in order to prevent severe respiratory tract infections in patients suffering from viral infection. Such results are obviously very promising as the mean duration of viral shedding in patients suffering from COVID-19 in China was at least 20 days and up to 37 days.8 In vitro studies has suggested that a possible mechanism of action of chloroquine (CQ) and HCQ could be the augmentation of the intracellular pH in acidic organelles such as endosomes and lysosomes, owing to the fact that both molecules are weak bases.9 Accordingly, it is known that acidic media (pH < 5) are mandatory for endosome maturation and function. CQ was thus reported to elevate the pH of lysosome form about 4.5 to 6.5 at 100 µM. Consequently, it could be surmised that endosome maturation might be blocked at intermediate stages of endocytosis, resulting in failure of further import of virions into the cytosol. Another possibility could be inhibition of SARS-CoV entry by CD or HCQ through their ability of changing the glycosylation state of ACE2 receptors and S-proteins. Accordingly, it has been checked using immunofluorescence analysis (IFA) and confocal microscopy that the transport of SARS-CoV-2 from early endosomes (EEs) to endolyosomes (ELs) required for the release of the viral genome, was blocked (in vitro) by CQ and HCQ.9 Another point concerns the high concentration of cytokines (IL1B, IFNγ, IP10, MCP1, MIP1A, TNFα) in the plasma of critically ill patients infected by SARS-CoV-2. As HCQ is a successful anti-inflammatory agent that has been extensively used in autoimmune diseases, one may anticipate its ability to decrease the production of cytokines and pro-inflammatory factors. It is also worth noting that if HCQ is less toxic than CQ, prolonged and/or overdose usage of both molecules may lead to poisoning with cardiovascular and renal complications. There is thus an obvious need of finding other much less toxic molecules. Another constraint should be the low price and the large-scale availability of theses molecules as on Friday March 27, the COVID-19 has affected 175 countries, with more than 535’000 confirmed cases and about 24’000 deaths worldwide.10
Methylene blue (MB⊕)
Cancer is another kind of disease able to lead to cytokines storms and one may expect a large number of deaths from COVID-19 in such patients. However, our survey among our database of patients treated with a combination of α-lipoic acid, hydroxycitrate and methylene blue suggests that this treatment prevents from severe infection from COVID-19. It may thus be anticipated, but yet not proved, that MB⊕ could be of considerable help for fighting against the COVID-19 epidemics. Here, we give a survey of the very interesting properties of this molecule with emphasis on chemistry, clinical trials being currently under investigation. Moreover, it is worth noting that methylene blue is the ancestor of modern anti-malaria drugs such as chloroquine and is associated to a lesser toxicity, the only drawback being a green-blue coloring of urine.
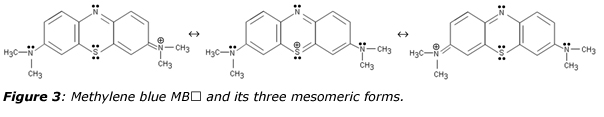
Methylene blue chloride is an old compound synthesized in 1876 by the German chemist Heinrich Caro, through oxidation of a mixture of dimethyl-4-phenylene-diamine Me2N-Ph-NH2 and hydrogen sulfide by ferric chloride:11
2 C8H12N2 + H2S + 6 FeCl3 = C16H18N3SCl + 6 FeCl2 + 4 HCl + NH4Cl
The sulfur atom bridges two molecules of the p-phenylene-diamine backbone (figure 3), forming a phenothiazine heterocyclic molecule displaying a formally positive thionium ion in one mesomeric form. Through aromatic resonance among the three fused rings, this formal positive charge may be delocalized over the two nitrogen atoms of the right and left dimethyl-amino groups leading a characteristic absorption at λ = 663 nm (ε = 75 mM-1·cm-1) for the un-protonated cation and undergoing a red-shift at λ = 740 nm for HMB2⊕ after protonation.12 Owing to strong absorption of the red part of the visible spectrum, cationic forms of methylene blue are deep-blue colored, a property used in 1882 by Robert Koch for staining the tubercle bacilli and extensively used by Paul Ehrlich (1854-1915) for differentiation between the different types of white blood cell.13,14 The chemical structure of methylene blue was established in 1884 and it was used in 1887 in combination with eosin by the Polish pathologist Czeslaw Checinski for evidencing presence of daisy-like (Plasmodium malariae) and sickle-shape (Plasmodium falciprum) parasites in blood smears. In 1891, Ehrlich discovered that methylene blue fell in the category of “magic bullets” drugs for its ability to target the malarial organism. Replacement of quinine, a natural substance derived from the cinchona tree of South America with very limited supply, by methylene blue, a costless synthetic dye has allowed large-scale production of antimalarial therapy. Subsequent developments of what has been called at that time “chemotherapy” has led to the synthesis in 1831 of the very successful drug quinacrine, marketed by Bayer under the names of mecaprine and atrabine. Atrabine was further modified in 1934 by the German chemist Hans Andersag by replacing the acridine ring with a quinolone ring, giving access to a product named “resochin” upon reaction of oxaloacetic acid diethylester with m-chloroaniline. But, the product was found to be too toxic for practical use in humans and in order to minimize toxicity; the compound 3-methylresochin, named “sontochin” was synthesized and patented in November 1939, after testing over 1’100 patients with malaria. In November 1945, E. K. Marshall rediscovered resochin giving to the compound its definitive name “chloroquin”, becoming the first-line antimalarial therapy for about 20 years, saving countless lives. Later on, hydroxychloroquine was developed and reported to be half as toxic as chloroquine.15
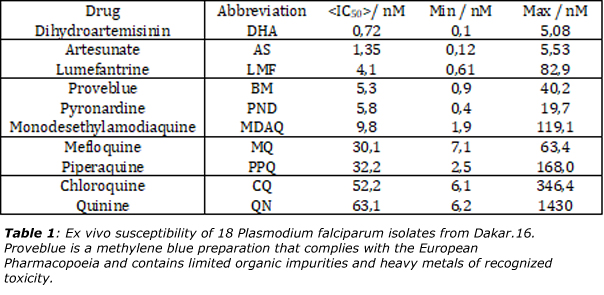
It follows that methylene blue may be considered as a template for the synthesis of substitutes of quinine in the cure of malaria. As shown in table 1, methylene blue is in fact in the top-five drugs against 18 stains of Plasmodium palcifarum.16 Moreover, a recent paper has evidenced that parasites responsible for malaria may also be hosts of yet unidentified RNA viruses,17 reinforcing the idea that it may exist a strong link between RNA viruses and anti-malaria drugs. There is also recent evidence that methylene blue activated with visible light effectively reduce Ebola Virus (EBOV), MERS-CoV, SARS-CoV, Crimean–Congo hemorrhagic fever virus (CCHFV) and Nipah virus (NiV) infectivity in platelets and plasma, respectively.18,19 It follows that methylene blue used in conjunction with light may open a quite novel route of anti-viral therapy by mixing biochemistry with photo-physics. The need for large-scale testing of methylene blue against COVID-19 is again reinforced.
 Methylene blue in non-viral pathologies
Methylene blue in non-viral pathologies
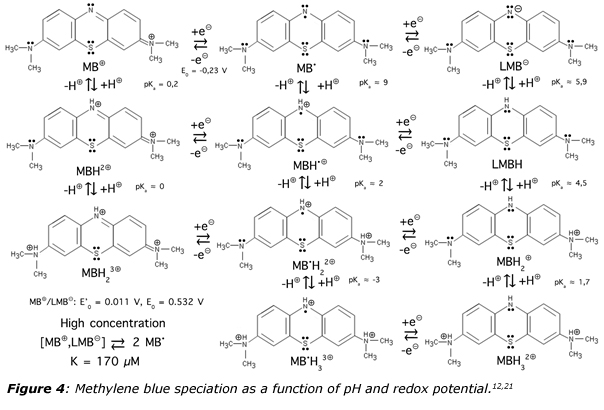
Methylene blue is in fact what may be called a BONARIA drug.20 Here the three letters “BON” means that it is a safe and efficacious remedy while the last letters indicates that it is also affordable (A), registered (R) and internationally accessible (IA). This comes from the quite peculiar chemical properties of this substance. As shown in figure 4, the behavior of methylene blue as a function of pH, redox potential and irradiation is quite diversified and fascinating.12,21 Upon assimilation, it is not a single molecule that enters blood circulation, but a full set of molecules, explaining the extreme versatility in a large number of pathologies ranging from microbiology to psychiatry. Concerning viral infections, it is worth noticing that when methylene blue undergoes a one-electron reduction, it becomes a neutral lipophilic MB• radical with good stability insured by its delocalization over several mesomeric forms. Moreover, such a radical acts as a weak base (pKa ≈ 9) favoring, as with chloroquine, transient alkalinization of cytosolic spaces.
Methylene blue also possesses antibacterial activity and is secreted in urine, explaining why it was heavily used for treating infections and painful disorder of the urinary tract in multi-ingredient prescriptions (polypharmacy).22 A quite useful combination was 5.4 mg of MB, 0.03 mg of atropine sulfate and 0.03 mg hyoscyamine (for pain relief of smooth muscle spasms), 40.8 mg of methamine (condensation product of formaldehyde with ammonia, breaking down in acidic urines), 5.4 mg of benzoic acid and 18.1 mg of phenyl salicylate (salol, an antiseptic). The mechanism of action of methylene blue against parasites has been partially elucidated involving homodimeric flavoenzymes of the glutathione (GR) reductase family that are present both in malarial parasite and the mammalian host cell.20 The first affected enzyme is glutathione reductase (GR) allowing reducing glutathione disulfide (GSSG) to the sulfhydryl form glutathione (GSH):
GR NADPH + H3O⊕ + GSSG = NADP⊕ + 2 GSH + H2O
The second enzyme is thioredoxin reductase (TrxR) allowing reduction of thioredoxins (Trx) that are proteins facilitating the reduction of other proteins by cysteine thiol-disulfide exchange:
TrxR NADPH + H3O⊕ + TrxS2 = NADP⊕ + 2 Trx(SH)2 + H2O
The role of GR and TrxR is to keep GSH and redoxins in the reduced state in order to maintain cytosolic spaces under reduced conditions. The key point is that methylene blue could be a substrate for TrxR with production of the reduced neutral and colorless form (leuco-methylene blue or LMBH) absorbing in the UV-part of the electromagnetic spectrum (λ = 340 nm, ε = 3.3 mM-1·cm-1 and λ = 258 nm, ε = 17.4 mM-1·cm-1):
TrxR NADPH + MB⊕ = NADP⊕ + LMBH
It follows that methylene acts as an inhibitor of the natural reactions of TrxR. It is worth noticing that methylene blue is unable to inhibit the enzyme dihydrolipoamide dehydrogenase (LipDH), another flavoprotein enzyme that oxidizes dihydrolipoamide to lipoamide (functional form of α-lipoic acid):
LipDH NAD⊕ + H2O + dihydrolipoamide(SH)2 = NADH + H3O⊕ + lipoamideS2
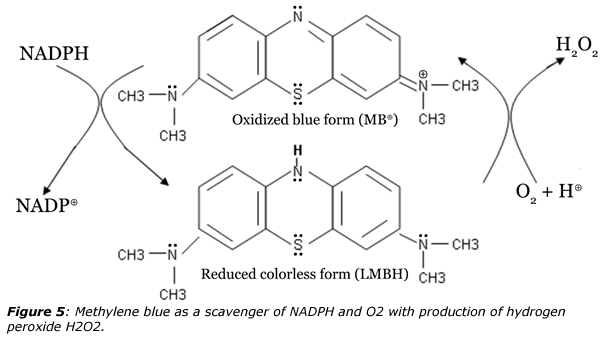
 However, the reduced form LMBH is unstable in presence of micromolar concentrations of molecular oxygen and auto-oxidizes readily under such conditions as shown in figure 5. The antioxidant thiol-producing enzymes guarding the reducing milieu of cytosolic spaces are thus turned in contact with methylene blue into pro-oxidant H2O2-producing enzymes challenging the reducing milieu that they are meant to protect. It follows that methylene blue is both an inhibitor and a subversive substrate allowing concomitant production of reactive oxygen species (ROS). Under such conditions, NADP(H) and molecular oxygen that are needed for the pathogen’s metabolism are irreversibly consumed by methylene blue. In addition, there is less GSH available in the parasite as a substrate of GSH S-transferase for the detoxification of hemes and other lipophilic compounds. The anti-bacterial effect of methylene blue then lies in the fact human GR reacts more slowly with methylene blue than parasites GR and that human TrxR is not present in erythrocytes. Accordingly, when parasitized red blood cells and normal erythrocytes are incubated together in MB-containing solution, the drug becomes concentrated selectively in the parasitized erythrocytes. A possible reason is that as LMBH bears no electrical charge, it easily permeates the membrane of digestive vesicles and is then auto-oxidized back to MB⊕, thus remaining trapped in the vesicles. It is finally worth noticing that synergistic effects have been found against P. falciparum in culture when using methylene blue in combination with artemisinin derivatives.20
However, the reduced form LMBH is unstable in presence of micromolar concentrations of molecular oxygen and auto-oxidizes readily under such conditions as shown in figure 5. The antioxidant thiol-producing enzymes guarding the reducing milieu of cytosolic spaces are thus turned in contact with methylene blue into pro-oxidant H2O2-producing enzymes challenging the reducing milieu that they are meant to protect. It follows that methylene blue is both an inhibitor and a subversive substrate allowing concomitant production of reactive oxygen species (ROS). Under such conditions, NADP(H) and molecular oxygen that are needed for the pathogen’s metabolism are irreversibly consumed by methylene blue. In addition, there is less GSH available in the parasite as a substrate of GSH S-transferase for the detoxification of hemes and other lipophilic compounds. The anti-bacterial effect of methylene blue then lies in the fact human GR reacts more slowly with methylene blue than parasites GR and that human TrxR is not present in erythrocytes. Accordingly, when parasitized red blood cells and normal erythrocytes are incubated together in MB-containing solution, the drug becomes concentrated selectively in the parasitized erythrocytes. A possible reason is that as LMBH bears no electrical charge, it easily permeates the membrane of digestive vesicles and is then auto-oxidized back to MB⊕, thus remaining trapped in the vesicles. It is finally worth noticing that synergistic effects have been found against P. falciparum in culture when using methylene blue in combination with artemisinin derivatives.20
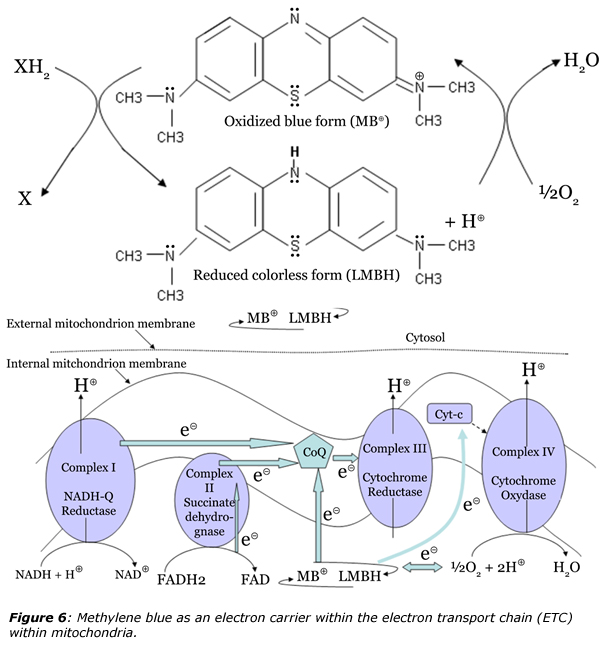
 In the 1920s methylene blue proved to be a dramatic antidote for carbon monoxide or cyanide poisoning.23 Consequently, methylene blue could be very efficient since 1940 for treatment of methemoglobinemia, a pathology where the ferrous ion of hemoglobin becomes oxidized into ferric ion, impairing attachment of dioxygen and thus reducing the oxygen-carrying capacity of the blood.22 Upon IV-injection methylene blue (1-2 mg·kg-1 or 1% sterile solution) transforms in contact with reductases in the erythrocytes into the colorless leuco-methylene blue (LMBH) able to reduce methemoglobin back to normal hemoglobin. It follows that if used as such rather low concentrations, methylene blue functions as an alternative electron carrier in mitochondria, which accepts electrons from NADH or FADH2 and transfers them to CoQ or cytochrome c and bypassing any complex I/III blockage (figure 6).24 It is worth noting than in this case, an harmless product, water, in produced instead of hydrogen peroxide (cf. figure 5).
In the 1920s methylene blue proved to be a dramatic antidote for carbon monoxide or cyanide poisoning.23 Consequently, methylene blue could be very efficient since 1940 for treatment of methemoglobinemia, a pathology where the ferrous ion of hemoglobin becomes oxidized into ferric ion, impairing attachment of dioxygen and thus reducing the oxygen-carrying capacity of the blood.22 Upon IV-injection methylene blue (1-2 mg·kg-1 or 1% sterile solution) transforms in contact with reductases in the erythrocytes into the colorless leuco-methylene blue (LMBH) able to reduce methemoglobin back to normal hemoglobin. It follows that if used as such rather low concentrations, methylene blue functions as an alternative electron carrier in mitochondria, which accepts electrons from NADH or FADH2 and transfers them to CoQ or cytochrome c and bypassing any complex I/III blockage (figure 6).24 It is worth noting than in this case, an harmless product, water, in produced instead of hydrogen peroxide (cf. figure 5).
 As a side effect, methylene blue is also able to scavenge any leakage in superoxide anion O2•⊝ from the ETC according to the reaction:
As a side effect, methylene blue is also able to scavenge any leakage in superoxide anion O2•⊝ from the ETC according to the reaction:
O2•⊝ + MB⊕ = O2 + MB•
2 MB• = MB⊕ + LMB⊝
Methylene blue is thus a potent antioxidant able stopping the oxidative cascade at its very beginning. It may thus also be considered as pyromaniac firefighter able to increase oxidative stress by accelerating ATP production and also able to eliminate the same oxidative stress thus generated. Associated to these well-known antioxidant properties is the ability of methylene blue for attenuating any ischemia/reperfusion injury through inhibition of superoxide generation by xanthine oxidase.25 The ability of methylene blue for minimization of free radical production in the mitochondrial ETC explains why it has positive effects under metabolically stressed conditions, such as ischemic brain injury, where excess free radicals may lead to cellular damage and cell death. Accordingly, in vivo studies have shown that methylene blue reaches its maximum concentration in blood by 5 min after intravenous administration in humans.26 The half-life of MB in blood after intravenous administration is 5.25 h in humans. No significant effects on vascular reactivity were observed using functional magnetic resonance imagery (fMRI) but a preferential potentiation of regions with a higher metabolic demand has been evidenced. Consequently, methylene blue concentrates in cortical regions with the largest metabolic energy demands in an activation task such as forelimb stimulation, boosting intellectual tasks.
Using methylene blue in patients with septicemia, leads to positive results owing to a direct inhibition of nitric oxide synthases (NOS), both constitutive and inducible. It inhibits guanylyl cyclase (GC) by binding to the heme group of the enzyme and blocks the catalytic functions of NO synthase by oxidation of the enzyme-bound ferrous iron.27 An additional effect on soluble guanylyl cyclase, which normally leads to the formation of cyclic guanosine monophosphate (cGMP) as the second messenger of NO•, adds to the inhibition of the NO–cGMP pathway. Methylene blue may be a more specific and potent inhibitor of NO synthase than guanylyl cyclase, because direct NO-donating compounds in the presence of methylene blue can still partially activate cGMP-signaling pathways. Methylene blue also inhibits platelet activation, adhesion, and aggregation synergistically with an inhibition of platelet thromboxane A2 and endothelial prostacyclin I2 production.27
A very important point is that if methylene blue is able to increase complex-IV activity by about 30%, it also has a marked hormetic action by capturing electrons of the ETC at high dose (> 10 mg/kg) and through interaction with nitrogen oxide synthase (NOS) may produces cardio-vascular effects.26 Studies performed in vitro has evidenced that complex-IV achieves a maximum activity for a 0.5 µM concentration. Above 5 µM, complex-IV activity is inhibited, and the larger the concentration, the stronger the inhibition.28 On the other hand, studies performed in vivo on rats has shown that a maximal locomotion activity was reached at a dose of 4 mg·kg-1, no effects being observed below 1 mg·kg-1 or above 10 mg·kg-1. Finally, above 50 mg·kg-1, locomotion activity becomes to be reduced.
Summing together all these properties explains why methylene blue could be of considerable use in neurodegenerative diseases29, ageing30, cancer31 or for healing psychic disorders.32 Figure 7 shows for instance how methylene blue may act on key enzymes involved in Alzheimer disease (AD). Beneficial effects of methylene blue against AD has been demonstrated in clinical studies and comes from the non-polar character of the reduced LMBH form allowing it to cross easily the blood-brain barrier, for hitting multiple molecular targets. Concerning the inhibition of Tau protein aggregation by methylene blue, an in vitro target seems to be the microtubule affinity-regulating kinase (MARK4) at Ser262, through stabilization of its dimeric form following cysteine oxidation. But, as shown in figure 7, this is just one of the mode of action, the other ones being the down-regulation of cholinesterase activity for preventing acetylcholine (ACh) degradation and the ability of scavenging superoxide. But research in this field is progressing quite rapidly. For instance, it was recently shown that methylene blue reverses Caspase-6-induced cognitive deficits by inhibiting Caspase-6, and Caspase-6-mediated neurodegeneration (inhibition of the cleavage of the amyloid precursor protein APP) and neuroinflammation.32 As Caspase-6-mediated damage seems to be reversible months after the onset of cognitive deficits suggesting that methylene blue could benefit Alzheimer disease patients by reversing Caspase-6-mediated cognitive decline.
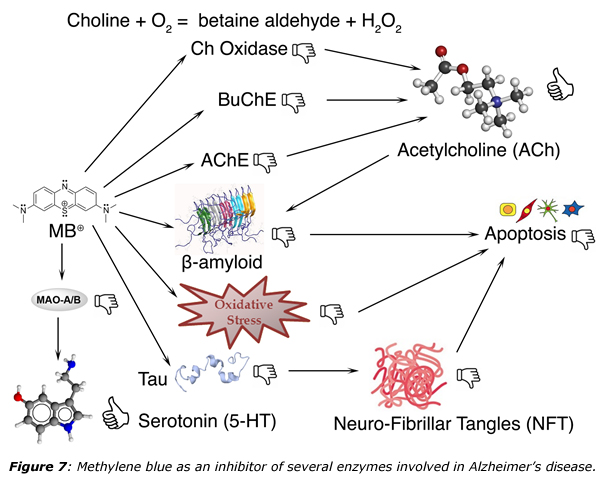
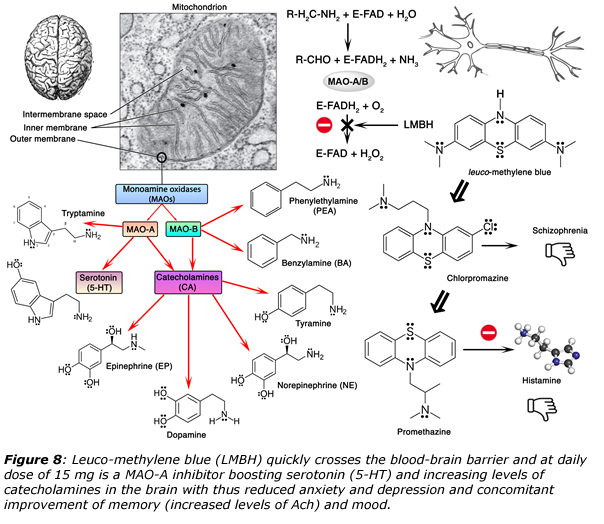
As shown in figure 7, methylene blue is also an inhibitor of monoamine oxidases (MAOs), a biochemical fact that has been known for many decades.32 Figure 8 gives more details about the antipsychotic effects of methylene blue that have been exploited for more than a century. Accordingly, many studies have confirmed that methylene blue influences neuronal communication by altering cholinergic, monoaminergic, and glutamatergic synaptic neurotransmission both in the central and the peripheral nervous systems. First, methylene blue is able to induce neuronal membrane depolarization with inhibition of Ca2⊕-activated K⊕ channels, activation of Ca2⊕ channels, and facilitation of Na⊕ channel inactivation.32 It also modulates the functions of various integral membrane proteins involved in transports of solutes such as glucose and ions such as Na⊕, K⊕, and H⊕. It was demonstrated that the cGMP pathway does not mediate the actions of methylene, reported in the majority of these studies.
Both glutamate and dopamine have been implicated in the pathogenesis of psychoses and methylene blue has been the lead compound for the development of classical antipsychotics. Thus, promethazine was made in the 1940s by a team of scientists from Rhône-Poulenc laboratories and shares with LMBH the same phenothiazine backbone and was the first-generation antihistaminic. It was used to treat allergies, trouble sleeping, and nausea and may help with some symptoms associated with the common cold. As it may also be used for sedating people who are agitated or anxious, it has led to the development of chlorpromazine that was first experimented in the early 1950s by the French military surgeon Henri Laborit in the hope of discovering a more effective anesthetic. While the drug did not cause his patients to lose consciousness, it did induce a remarkable calmness. This discovery was the very beginning of the “chemical lobotomy” revolution for the treatment of both acute and chronic psychoses, including schizophrenia and the manic phase of bipolar disorder, as well as amphetamine-induced psychosis. Considering the similarities in the chemical structures of methylene blue and antipsychotics of, it is likely that methylene blue modulates the activity of dopamine receptors. And owing to its antioxidant properties, it could also be of considerable help in the prevention of Li-toxicity in bipolar disorder patients.
 Among other interesting properties, methylene blue has been shown to modulate the physiological actions of hormones involved in the hypothalamo-pituitary-peripheral axis, increasing thyroid peroxidase activity and enhancing the iodination of thyronines, with subsequent increases in the synthesis of thyroxine.32 Moreover, when exposed to light, MB becomes photosensitized, leading to the release of cytotoxic, highly active, and short-lived oxygen-derived species such as singlet oxygen 1O2. It has also been shown that photons in the red-to-near-infrared frequency range of approximately 620–1150 nm penetrate to the brain and intersect with the absorption spectrum of cytochrome oxidase.34 While low-dose methylene blue and low-level near-infrared light may produce different pleiotropic cellular effects, both interventions cause a similar up-regulation of mitochondrial respiration with similar benefits to protect nerve cells against degeneration. These human studies suggest that low-dose methylene blue may have potential therapeutic applications in neurology as a neuroprotective agent, and in psychiatry and clinical psychology to facilitate psychotherapeutic interventions. Similarly, low-level near-infrared light improved human neurological outcome after ischemic stroke,35 and could help in conjunction with methylene blue to enhance emotional and neurocognitive functions such as sustained attention and working memory in humans.
Among other interesting properties, methylene blue has been shown to modulate the physiological actions of hormones involved in the hypothalamo-pituitary-peripheral axis, increasing thyroid peroxidase activity and enhancing the iodination of thyronines, with subsequent increases in the synthesis of thyroxine.32 Moreover, when exposed to light, MB becomes photosensitized, leading to the release of cytotoxic, highly active, and short-lived oxygen-derived species such as singlet oxygen 1O2. It has also been shown that photons in the red-to-near-infrared frequency range of approximately 620–1150 nm penetrate to the brain and intersect with the absorption spectrum of cytochrome oxidase.34 While low-dose methylene blue and low-level near-infrared light may produce different pleiotropic cellular effects, both interventions cause a similar up-regulation of mitochondrial respiration with similar benefits to protect nerve cells against degeneration. These human studies suggest that low-dose methylene blue may have potential therapeutic applications in neurology as a neuroprotective agent, and in psychiatry and clinical psychology to facilitate psychotherapeutic interventions. Similarly, low-level near-infrared light improved human neurological outcome after ischemic stroke,35 and could help in conjunction with methylene blue to enhance emotional and neurocognitive functions such as sustained attention and working memory in humans.
Conclusion
With the current COVID-19 outbreak, the world is facing a challenge and every possibility of helping people should be considered. Our preliminary data suggest but do not prove that Methylene blue might be a good treatment for influenza- like illnesses. Both Methylene blue and its derivatives such as chloroquine may share similar mechanism of action. Time is ripe for a prospective randomized clinical trial for the treatment of this dreadful disease.
Old drugs which have been tested for other indications ( such as Methylene blue or Chloroquine) have a well-defined safety profile. They are often more effective than new drugs from the High Tech. The Covid 19 epidemics like cancer has a solution. Reporpusing of known molecules will help cure these deadly diseases.
References
- R. Jonsdottir, R. Dijkman, Virology J., 2016, 13, 24.
- S. Kahn, K. McIntosh, The Pediatric Infectious Disease Journal, 2005, 24, S223.
- R. Fehr, S. Perlman, Methods Mol. Biol., 2015 ; 1282: 1–23.
- G. Andersen, A. Rambaut, W. I. Lipkin, E.C. Holmes, R. F. Garry, 2020, Nature Medicine, https://doi.org/10.1038/s41591-020-0820-9.
- Owczarek, A. Szczepanski, A. Milewska, Z. Baster, Z. Rajgur, M. Sarn, K. Pyrc, 2018, Scientific Reports, 8:7124.
- Fang, G. Karakiulakis, M. Roth, 2020, Lancet RespirMed, March 11, https://doi.org/10.1016/S2213-2600(20)30116-8.
- Gautret, J.-C. Lagier, P. Parola, V. T. Hoang, L. Medded, M. Mailhe, B. Doudier, J. Courjon, V. Giordanengo, V. E. Vieira, H. Tissot-Dupont, S. Honoré, P. Colson, E. Chabrière, B. La Scola, J.-M. Rolain, P. Brouqui, D. Raoult, 2020, International Journal of Antimicrobial Agents – In Press , March 17 – DOI : 10.1016/j.ijantimicag.2020.105949.
- Zhou, T. Yu, R. Du, G. Fan, Y. Liu, Z. Liu, J. Xiang, Y. Wang, B. Song, X. Gu, L. Guan, Y. Wei, H. Li, X. Wu, J. Xu, S. Tu, Y. Zhang, H. Chen, B. Cao, 2020, Lancet, March, 9. https://doi.org/10.1016/ S0140-6736(20)30566-3.
- Liu, R. Cao, M. Xu, X. Wang, H. Zhang, H. Hu, Y. Li, Z. Hu, W. Zhong, M. Wang, 2020, Cell Discovery, 6 : 16. DOI : https://doi.org/10.1038/s41421-020-0156-0.
- Dong, H. Du, L. Gardner, 2020, Lancet Infect. Dis., February 19, https://doi.org/10.1016/ S1473-3099(20)30120-1. For interactive dashboard of global COVID-19 cases see https://arcg.is/0fHmTX.
- Caro, 1878, US Patent n°204,796; june, 11.
- Contineanu, C. Bercu, I. Contineanu, A. Neacsu, 2009, Analele Universităţii din Bucureşti – Chimie (serie nouă), 18: 29.
- C. Cook, 1997, J. Clin. Pathol., 50: 716.
- Krafts, E. Hempelmann, A. Skorska-Stania, 2012, Parasitol. Res., 111 : 1.
- C. Warhurst, J. C. P. Steele, I. S. adagu, J. C. Craig, C. Cullander, 2003, J. Antimicrobial Chemotherapy, 52: 188.
- Fall, C. Camara, M. Fall, A. Nakoulima, P. Dionne, B. Diatta, Y. Diemé, B. Wade, B. Pradines, 2015, Malaria Journal, 14, 60.
- Charon, M. J. Grigg, J.-S. Eden, K. A. Piera, H. Rana, T. William, K. Rose, M. P. Davenport, N. A. Anstey, E.C. Holmes, 2019, PLoS Pathog., 15 : e1008216, https://doi.org/10.1371/journal.ppat.1008216.
- Eickmann, U. Gravemann, W. Handke, F. Tolksdorf, S. Reichenberg, T. H. Müller, A. Seltsam, 2018, Transfusion, 58: 2202.
- Eickmann, U. Gravemann, W. Handke, F. Tolksdorf, T. H. Müller, A. Seltsam, 2020, Vox Sanguinis, DOI: 10.1111/vox.12888.
- Buchholz, R. H. Schirmer, J. K. Eubel, M. B. Akoachere, T. Dandekar, K. Becker, S. Gromer, 2008, Antimicrobial agents and Chemotherapy, 52: 183.
- Impert, A. Katafias, P. Kita, A. Mills, A. Pietkiewicz-Graczyk, G. Wrzeszcz, 2003, Dalton Trans., 348-353.
- Scheindlin, 2008, Molecular interventions, 8: 268.
- Miclescu, L. Wiklund, 2010, J. Rom. Anest. Terap. Int., 17:35.
- Wen, W. Li, E. C. Poteet, L. Xie, C. Tan, L.-J. Yan, X. Ju, R. Liu, H. Qian, M. A. Marvin, M. S. Goldberg, H. She, Z. Mao, J. W. Simpkin, S.-H. Yang, 2011, J. Biol. Chem., 286: 16504.
- C. Salaris, C. F. Babbs, W. D. Voorhees III, 1991, Biochemical Pharmacology, 42: 499.
- Huang, F. Du, Y.-Y. I. Shih, Q. Shen, F. Gonzalez-Lima, T. Q. Duong, 2013, Neuroimage, 72: 237.
- Wiklund, S. Basu, A. Miclescu, P. Wiklund, G. Ronquist, H. S. Sharma, 2007, Ann. N. Y. Acad. Sci., 1112:231.
- K. Bruchey and F. Gonzalez-Lima, 2008, Am. J. Pharmacol. Toxicol., 3 : 72.
- Li, H. Yang, Y. Chen, H. Sun, 2017, Eur. J. Med. Chem., 132: 294.
- -M. Xiong, M. O’Donovan, L. Sun, J. Y. Choi, M. Ren, K. Cao, 2017, Scientific Reports, 7: 2475.
- S. G. Barron, 1930, J. Exp. Med., 52 : 447.
- Oz, D. E. Lorke, M. Hasan, G. A. Petroianu, 2010, Medicinal Research Reviews, 21:93
- Zhou, J. Flores, A. Noël, O. Beauchet, P. J. Sjöström, A. C. LeBlanc, 2019, Acta Neuropathologica Communications, 7: 210.
- Gonzalez-Lima, A. Auchter, 2015, Frontiers in Cellular Neurosciences, 9: 179
- Lampl, J. A. Zivin, M. Fisher, R. Lew, L. Welin, B. Dahlof, P. Borenstein, B. Andersson, J. Perez, C. Caparo, S. Ilic, U. Oron, 2007, Stroke, 38: 1843.
- Pietkiewicz-Graczyk, O. Impert, A. Katafias, P. Kita, 2003, Polish J. Chem., 77: 475.




Has there been any follow up on these patients?
Des mises à jour sur les patients?
Bonjour,
Ces travaux montrent que la piste antibactérienne tient la route
Tout est expliqué dans cet article
recherches cliniques en Lorraine
mes travaux théoriques
https://www.agoravox.fr/tribune-libre/article/des-medecins-ont-trouve-un-223202
Comme toujours, la publication est très intéressante.
Malheureusement je constate encore, que c’est bourré de fautes d’orthographe, ponctuation, grammaire et vocabulaire ! (Oui, je suis exaspérée, ça déborde des ridelles)
A toute l’équipe autour de Laurent Schwartz,
vous faites un travail scientifique, de recherche formidable ; vous êtes apparemment très dévoués et je vous en remercie.
Je ne suis pas aussi intelligente que vous, ni suffisamment instruite, pour lire et comprendre en première lecture vos publications. Par endroit, je fais des hypothèses de correction et vérifie laquelle est cohérente avec la suite…
Certes, pour moi c’est avantageux de tenter de corriger le texte : j’apprends d’autant mieux le sujet !!! Mais est-ce votre objectif ?
J’espère que vous êtes plus sérieux dans les recherches que dans la rédaction du rapport ( euh… quel sérieux dans les mesures et leur enregistrement ?)
Sérieusement, pour qui écrivez vous ??? Quel respect voulez-vous afficher vis à vis de vos pairs , vis à vis du public ?
A votre disposition.
Bravo de faire étalage de votre connaissance de la langue anglaise, mais le sujet actuel n’est pas vraiment l’orthographe, la syntaxe ou la ponctuation, il y a des choses plus urgentes à régler et ce rapport n’a pas vocation à devenir une œuvre littéraire, ce n’est qu’une modeste contribution aux recherches multiples qui occupent beaucoup de scientifiques et chercheurs en ce moment. Nous aurions apprécié que vos remarques portent sur le fond plutôt que la forme.
Bien à vous.
?????? Etalage de ma connaissance de la langue anglaise ???? Euh … Moi pas bilingue justement !
Pour des remarques sur le fond, je reviendrai (peut-être) quand j’aurai fini de décrypter et tout bien compris.
J’ai des questions que je brûle de poser sur ce texte, sur celui de SCOT, sur celui de l’entropie et de la vie… des questions en lien avec la vie sur le terrain. Au point de lecture où j’en suis, je n’ose pas… Je me refuse de faire perdre du temps à Marc (pour cette publication, idem avec les autres)… et si la réponse était déjà dans le texte mais je n’ai pas décrypté correctement donc pas compris…?
Je me demande vraiment si je suis le bon public !!! Pas assez instruite (comme je l’ai dit précédemment) ??? Clairement hors du « milieu » ??? Beaucoup trop concernée par le sujet ???
Advienne que pourra à tous les malades qui n’y comprennent rien ou presque.
Merci à la fabuleuse équipe de chercheurs pour ses travaux ; j’y vois des raisons d’espérer.
Etant atteint d’un myélome multiple c’est une très bonne nouvelle pour tous les malades sous traitement métabolique et bleu de méthylène leur donnant probablement une très bonne protection contre le Covid 19. Je suis admiratif et très reconnaissant pour tout le travail de recherche, de persévérance du Dr. Laurent Schwartz. Un grand espoir pour les malades du cancer (et autres) et maintenant un immense espoir pour guérir les malades du Covid 19. Un bel exemple qui devrait interroger les Pharma qui dépensent des milliards dans la recherche (pour des médicament hors prix) alors que des traitements efficace et abordable existent. Mes plus vives remerciements,
Pierre Guthauser, Meyrin, Suisse
Etant atteint d’un myélome multiple c’est une très bonne nouvelle pour tous les malades sous traitement métabolique et bleu de méthylène leur donnant probablement une très bonne protection contre le Covid 19. Je suis admiratif et très reconnaissant pour tout le travail de recherche, de persévérance du Dr. Laurent Schwartz. Un grand espoir pour les malades du cancer et maintenant un immense espoir pour guérir les malades atteint du Covid 19. Un bel exemple qui devrait interroger les Pharma qui dépensent des milliards dans la recherche (et produisent des médicaments hors prix) alors que des traitements efficace et abordable existent. Tous mes vives remerciements.
Pierre Guthauser, Meyrin (Suisse).
Bravo pour votre persévérance dans ces champs de recherche.
Grâce à vous, on se sent plus fort en tant que patient
Cordialement
Laurence
Butterfly Warrior
L’action anti-virale du Bleu de Méthylène est bien connue (en plus d’autres bienfaits). Mais elle ne coûte pas cher et ne rapporte rien à Big Pharma. Je propose de contacter le Docteur Raoul de l’informer de cette étude. Par ailleurs faites diffuser cette information au maximum (en Europe et ailleurs). Ceci nous aidera à faire connaître le traitement métabolique.
Bravo et Bonne continuation